@Akhunter I understand your concern given it's not as common (yet) and the fact that there is a lot of data supporting pharmaceutical applications with say cotton seed oil etc but that is with most things. There is a tipping point where an older method/product is improved and data is built against the new method/product to support or disprove the advances and/or benefits of the shift. This can result in failure but in a lot of cases things come out ahead as an advancement to current process.
I pulled in a number of medical research and pharmaceutical data sources, into Grok, comparing the common carrier oils, use by pharmaceutical companies over time including data seen in medical research on carrier oil benefits and risks.
This is summarized in a table below but I've also pasted the data leveraged to build this table at the end of this post. Couple key items stuck out to me was the shift by pharmacy to Mig840 and the health risks with all of the oils (Mig840 seemingly to have the least reported). So to your point there is a lot of data on prior carrier oils, but that data is only continuing to build out further with additional data supporting Mig as it is being leveraged further in the field.
View attachment 205214
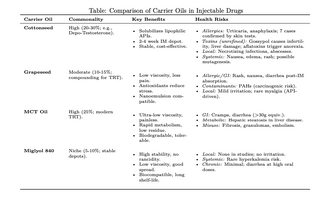
Summary of Common Carrier Oils in Injectable Drugs
Carrier oils play a critical role in the formulation of injectable drugs, particularly for lipophilic active pharmaceutical ingredients (APIs) that require solubilization for intramuscular (IM) or subcutaneous (SC) administration. These oils act as vehicles to ensure stability, controlled release, and biocompatibility, often forming depots that provide sustained drug delivery over days to weeks. In pharmaceutical and compounding pharmacy settings, the choice of carrier oil balances factors like viscosity (for ease of injection), solubility enhancement, and tissue tolerability. Common oils include sesame, soybean, and olive oils, but the focus here is on cottonseed oil, grapeseed oil, medium-chain triglycerides (MCTs), and Miglyol 840 (a propylene glycol monocaprylate, a semi-synthetic MCT variant).
Pharmaceutical companies frequently use these oils in commercial products, with cottonseed oil being one of the most established (e.g., in Pfizer's Depo-Testosterone for testosterone cypionate). Grapeseed oil is more prevalent in compounding pharmacies for custom testosterone replacement therapy (TRT) formulations due to its thinner profile. MCTs, including variants like Miglyol 840, are increasingly adopted for their low viscosity and rapid metabolism, appearing in modern TRT injectables and specialized depots for antipsychotics or hormones. Usage data from industry analyses indicate cottonseed oil in ~20-30% of oil-based IM formulations, grapeseed in ~10-15% (mostly compounded), MCTs in ~25% and rising, and Miglyol 840 in niche pharma applications (~5-10%) for high-stability needs. These oils are GRAS (Generally Recognized as Safe) by the FDA when refined, but risks arise from impurities, allergies, or misuse.
Cottonseed Oil
Cottonseed oil, derived from Gossypium seeds, is a longstanding pharmaceutical excipient refined to remove toxins like gossypol. It is highly common in commercial injectables, used by major firms like Pfizer for sustained-release depots in hormone therapies.
Benefits: Provides excellent solubilization for highly lipophilic drugs, enabling 2-4 week sustained release via IM depot formation as the oil slowly diffuses in muscle tissue. It offers chemical stability, compatibility with microemulsions for enhanced penetration, and cost-effectiveness in large-scale production.
Health Risks: Unrefined forms contain gossypol, aflatoxins, and cyclopropenoid fatty acids, linked to infertility (spermatogenesis inhibition in males), liver damage (elevated enzymes, hepatotoxicity), pregnancy complications (embryotoxicity), respiratory distress, and anorexia. Case reports document hypersensitivity: seven patients experienced systemic reactions (urticaria, angioedema, anaphylaxis) after ingesting supplements, with skin testing confirming IgE-mediated allergy to cottonseed protein; similar risks apply to injectables, exacerbating allergic rhinitis or asthma. Accidental veterinary injections (e.g., ceftiofur in cottonseed oil) caused deep infections, necrosis, and recurrent abscesses requiring debridement and antibiotics. Local IM reactions include pain, erythema, swelling, nodules, and furuncles; high-dose anabolic misuse led to elevated liver enzymes, nausea, edema, palpitations, rash, fatigue, and reduced appetite. Reproductive studies show no direct injection effects, but chronic exposure risks mutagenesis or aberrant crypt foci in the colon.
Grapeseed Oil
Extracted from Vitis vinifera seeds, grapeseed oil is less ubiquitous than cottonseed but favored in compounding for its lightness, appearing in ~10-15% of custom steroid injectables.
Benefits: Low viscosity (~28 cP) minimizes injection pain and allows smaller-gauge needles; rich in antioxidants (e.g., proanthocyanidins) that may reduce oxidative stress at injection sites. It enhances transdermal penetration in nanoemulsions and supports cardioprotective effects in adjunctive therapies.
Health Risks: Generally low risk profile, with few injection-specific cases; however, potential polycyclic aromatic hydrocarbon (PAH) contamination in poorly processed oils poses carcinogenic concerns. Oral supplement cases report recurrent nausea, vomiting, diarrhea, and acute weakness, potentially extrapolating to systemic absorption post-injection. Allergic reactions include rash, itching, swelling, dizziness, and breathing difficulties; one compounded TRT formula warned of heart/kidney/liver exacerbation. Animal studies show no major toxicities, but human patch tests note mild irritation. In statin nanoemulsions, indirect risks like myalgia or hepatotoxicity stem from the API, not the oil. Overall, adverse events are rare (<1% in user reports), but monitoring for GI upset (diarrhea, bloating) is advised.
Medium-Chain Triglycerides (MCT Oil)
MCTs, typically C8-C10 fatty acids from coconut or palm kernel, are surging in popularity for injectables, used in ~25% of modern TRT formulations by compounding pharmacies and pharma innovators.
Benefits: Ultra-low viscosity (~30 cP) enables painless, rapid injections with minimal tissue residue; quick hepatic metabolism avoids long-term depots, reducing irritation while providing immediate bioavailability. Non-toxic in acute tests, with low ocular/dermal irritation and biodegradability.
Health Risks: Primarily GI-related: abdominal cramps, diarrhea, bloating, and nausea at high doses (>30g/day equivalent), due to rapid absorption overwhelming liver processing; rare fat accumulation in the liver (hepatic steatosis) in predisposed individuals. Allergic reactions manifest as hives, swelling, or anaphylaxis. In non-pharma misuse (e.g., bodybuilding oil injections), cases report irreversible muscle damage, paraffinomas (sclerosing lipogranulomas), ulcerations, infections, embolism, and fibrosis; one report detailed swelling, tenderness, and foreign-body granulomas post-IM injection. Pharma-grade use shows low toxicity, but diabetes/liver disease patients risk exacerbated issues. No reproductive/organ toxicities in animals, but start low to mitigate overeating from hunger hormone stimulation.
Miglyol 840
A synthetic propylene glycol diester (C8/C10), Miglyol 840 is a pharma-grade excipient used in ~5-10% of specialized IM formulations for its polarity and stability.
Benefits: Exceptional oxidative/thermal stability prevents rancidity, ideal for long-shelf-life injectables; low-medium viscosity (~13-15 cP) and high spreading facilitate even drug distribution. Biocompatible with poorly soluble APIs, enhancing solubility without irritation; fully metabolizable, reducing residue risks.
Health Risks: Minimal reported; animal toxicity studies (rat/monkey) show no injection-site reactions, histopathological changes, or systemic effects, even at high doses. Derived from propylene glycol, low oral exposure risks (e.g., no neuro/nephrotoxicity) apply, but injectables amplify potential for hyperkalemia or hypertension if API-interacting. No case reports of adverse events; human patch tests confirm no erythema/edema. Biodegradability minimizes environmental/long-term concerns, with only theoretical GI depression at extreme oral doses (>50,000 ppm in rats). Overall, considered a benchmark for safety in injectables.