- Joined
- Aug 20, 2013
- Messages
- 27,526
- Reaction score
- 18,153
- Points
- 121
Eating for recovery before bed..Facts vs Myths
I just wanted to put this out there, because to many bro's advocate-
"Long as you hit your required protein macros before bed then there is no need to take it before bed.""
- Not entirely true, but there's some truth to that, but other benefits also exist with slow absorption proteins while resting/recovering/sleep
There is some absolute truth with consuming slow/fast acting proteins.
The speed of absorption of dietary amino acids varies according to the type of ingested dietary protein... Learn just how important slow and fast proteins, taken at the appropriate time, can affect your ability to put on lean mass.Many people avoid eating right before bed as they fear that the calories are more likely to be stored as fat. This is not the case though. Your body doesn't have an on-off switch and you still burn calories while you sleep. According to the American Dietetic Association, it's excess calories that determine whether you gain weight, not when you eat them. Too many calories at breakfast or lunch will be just as detrimental as too many calories right before bed.
Premise
As much as we may think of bodybuilding as a cloistered subculture, we are forever bombarded with training and nutritional tips from sources far removed from squat racks and posing daises. So it is with this axiom, which is such a ubiquitous feature of the sort of diets Oprah hypes that many beginning bodybuilders dare not breach it, and it breeds confusion about what and when to eat to gain only muscle and not fat
Science
When you sleep, you?re on a fast. During that fast, your body is forced to turn to your own muscle protein for fuel, converting those amino acids into glucose. In other words, while you?re in dreamland, you?re experiencing the nightmare of cannibalizing your own muscles. The longer you go before sleep without eating, the more your muscle will be eaten away. That?s why we always recommend that you end your day with a slow-digesting protein, such as a casein protein shake or cottage cheese.
(Research from Groups located in Texas, and even the Netherlands discovered that trained bodybuilders drinking a casein protein shake right before bed for eight weeks gained significantly more muscle than those who consumed the same casein shake in the middle of the day.)
Verdict
We started with the easiest myth to shoot down, for not only is it OK to chow down long after sundown, it?s crucial to eat a protein meal immediately before going to bed in order to feed your muscles the nutrients they need to recover and grow while you sleep. Go with 20-40 grams of slow-digesting protein, such as a casein shake or cottage cheese. If you?re trying to pack on mass and don?t store fat easily, take your protein with about 20-40 g of slow-digesting carbs, such as oatmeal, sweet potatoes or whole-wheat bread.
Now with this being said:
Read the study and see the charts in regards to why a slow releasing protein has a full advantage (pre-sleep)
Slow and fast dietary proteins differently modulate postprandial protein accretion. Boirie Y, et al. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14930-5.Full text at:
Proc. Natl. Acad. Sci. USA
Vol. 94, pp. 14930?14935, December 1997
Physiology
Slow and fast dietary proteins differently modulate postprandial
protein accretion
(amino acid turnoverypostprandial protein anabolismymilk proteinystable isotopes)
YVES BOIRIE*, MARTIAL DANGIN*?, PIERRE GACHON*, MARIE-PAULE VASSON?, JEAN-LOUIS MAUBOIS?,
AND BERNARD BEAUFRE`RE*?
*Laboratoire de Nutrition Humaine, Universite? Clermont Auvergne, Centre de Recherche en Nutrition Humaine, BP 321, 63009 Clermont-Ferrand Cedex 1,
France; ?Nestec, Ltd., Nestle? Research Center, P.O. Box 44, CH 1000 Lausanne 26, Switzerland; ?Laboratoire de Biochimie, Biologie Mole?culaire et Nutrition,
Universite? Clermont Auvergne, BP 38, 63001 Clermont-Ferrand Cedex 1, France; and ?Laboratoire de Technologie Laitie`re, Institut National de la Recherche
Agronomique, 35042 Rennes Cedex, France
Communicated by John Waterlow, University of London, London, United Kingdom, October 7, 1997 (received for review April 20, 1997)
In relation to the 1997 Boirie study, Lyle summarized that the researchers found the following: whey spiked blood amino acid levels faster than casein, but blood amino acid levels dropped more quickly as well. Casein, in contrast, took much longer to digest, actually providing amino acids for around 8 hours to the body ... Both casein and whey hit the bloodstream at about the same time (about an hour in), that is, whey didn?t actually get into the system faster. However, whey spiked blood amino acid levels higher at that one hour point.
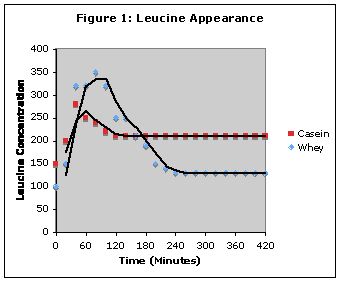

ABSTRACT The speed of absorption of dietary amino
acids by the gut varies according to the type of ingested dietary
protein. This could affect postprandial protein synthesis,
breakdown, and deposition. To test this hypothesis, two intrinsically
13C-leucine-labeled milk proteins, casein (CAS)
and whey protein (WP), of different physicochemical properties
were ingested as one single meal by healthy adults.
Postprandial whole body leucine kinetics were assessed by
using a dual tracer methodology. WP induced a dramatic but
short increase of plasma amino acids. CAS induced a prolonged
plateau of moderate hyperaminoacidemia, probably
because of a slow gastric emptying. Whole body protein
breakdown was inhibited by 34% after CAS ingestion but not
after WP ingestion. Postprandial protein synthesis was stimulated
by 68% with the WP meal and to a lesser extent (131%)
with the CAS meal. Postprandial whole body leucine oxidation
over 7 h was lower with CAS (272 6 91 mmolzkg21) than with
WP (373 6 56 mmolzkg21). Leucine intake was identical in
both meals (380 mmolzkg21). Therefore, net leucine balance
over the 7 h after the meal was more positive with CAS than
with WP (P < 0.05, WP vs. CAS). In conclusion, the speed of
protein digestion and amino acid absorption from the gut has
a major effect on whole body protein anabolism after one
single meal. By analogy with carbohydrate metabolism, slow
and fast proteins modulate the postprandial metabolic response,
a concept to be applied to wasting situations.
Dietary carbohydrates are commonly classified as slow and fast
because it now is well recognized that their structure affects
their speed of absorption, which in turn has a major impact on
the metabolic and hormonal response to a meal (1). On the
other hand, little is known about whether postprandial protein
kinetics are affected by the speed of absorption of dietary
amino acids; the latter is very variable, depending on gastric
and intestinal motility, luminal digestion, and finally mucosal
absorption. This lack of data is due to the fact that postprandial
amino acid kinetics have been studied almost exclusively
during continuous feeding, obtained either by a nasogastric
infusion or by small repeated meals (2?7). Measurements are
done 2?4 h after initiation of feeding, once isotopic and
substrate steady-state is achieved. Under these conditions, any
difference related to the speed of dietary amino acid absorption
is blunted.
There is, however, indirect evidence that this parameter
might be of importance. Indeed, the postprandial amino acid
levels differ a lot depending on the mode of administration of
a dietary protein; a single protein meal results in an acute but
transient peak of amino acids (9?11) whereas the same amount
of the same protein given in a continuous manner, which
mimics a slow absorption, induces a smaller but prolonged
increase (12). Amino acids are potent modulators of protein
synthesis, breakdown, and oxidation, so such different patterns
of postprandial amino acidemia might well result in different
postprandial protein kinetics and gain. Of interest, whole body
leucine balance, an index of protein deposition, was shown
recently to differ under these two circumstances (13).
Therefore, our hypothesis was that the speed of absorption
by the gut of amino acids derived from dietary proteins might
affect whole body protein synthesis, breakdown, and oxidation,
which in turn control protein deposition. To test this hypothesis,
we compared those parameters, assessed by leucine
kinetics, after ingestion of a single meal containing either whey
protein (WP) or casein (CAS), taken as paradigms for ??fast??
and ??slow?? proteins, respectively. Indeed, WP is a soluble
protein whereas CAS clots into the stomach, which delays its
gastric emptying and thus probably results in a slower release
of amino acids (14). Speed of amino acid absorption was
directly assessed by using a newly developed tracer, i.e., milk
protein fractions intrinsically labeled with L-[1-13C]leucine
(15). Leucine kinetics were modelized by using non-steadystate
equations as recently described (16). Our results demonstrate
that amino acids derived from CAS are indeed slowly
released from the gut and that slow and fast proteins differently
modulate postprandial changes of whole body protein
synthesis, breakdown, oxidation, and deposition.
I just wanted to post some of the study.. I will include the hyper link below that provides full detail,in regards to fast/slow proteins/and aminos..
(Slow and fast dietary proteins differently modulate postprandial protein)
Now with this being said more reads in regards-
ne)levels peak during sleep. 75 percent of daily hGH output is produced nse to get all the nutrients you'll need, then let the GH do
tNow what protein is the best to
consume prior to sleeping?
"Casein "
Keep reading
What is casein protein?
Casein (pronounced kay-seen) is the predominant protein found in milk. It is made by separating the casein from the whey in dairy (milk protein is 80 percent casein and 20 percent whey). There are three main types of casein protein: micellar casein, milk protein isolate and calcium caseinate. On average, one scoop (30 grams) of casein protein powder has approximately 100-120 calories and 25 grams of protein.
Besides its slow-digesting benefits, casein is invaluable for its high glutamine content. Of all the protein powders available, casein has the highest concentration of this amino acid. Glutamine provides a multitude of functions, which include increasing levels of the branched chain amino acid leucine in muscle fibers, enhancing protein synthesis and therefore, muscle growth. Because the immune system requires glutamine to function, consuming extra glutamine prevents the immune system from stealing it from muscle fibers, further averting catabolism. Glutamine also boosts growth hormone levels and can even aid fat loss by increasing the amount of calories and body fat burned both at rest and during exercise.
Do blended proteins such as whey offer the same effect as straight casein?
Either protein supplements are straight whey, soy, egg or casein; or they are a combination of any or all of these kinds of proteins, making them blends. What can a blend of proteins offer that a straight protein cannot? Basically, different rates of digestion. This means you can take a blended protein any time to get quick, medium, and prolonged absorption of protein.
But, I really like my ?whey? protein supplement.
Turn your favorite whey protein shake into a slow digesting one by simply mixing with milk, preferably low fat or skimmed. While casein protein is ?optimal? before bed, don?t forget that milk is 80 percent casein, adding it to any whey protein will slow down its absorption. Adding a fat such as natural peanut butter, flax or other healthy fat can further slow digestion, thus ?mimicking? casein protein.
To close the read and get to the conclusion here..
Don't deprive yourself, and fall for the hoopla that if you consume your proper intake of macro's that you don't need protein prior to rest/recovery(sleep)..
It most certainly won't hinder your gains on a large scale,but it surely is more beneficial than going without!
Regards,
Vision
____________________________
More info
BCAA's before bed is it necessary?
Truth is BCAA's need to be avail for use along with protein many researches
have stated that low to no protein before bed yields a low protein synthesis rate while sleeping,
you do burn cals when sleeping, you ever wake up lighter in the AM???
Its been proven that it will yield an add advantage with keeping plasma BCAA's levels higher throughout the state of rest/sleep/night
Pre-Sleep Protein Ingestion to Improve the Skeletal Muscle Adaptive Response to Exercise Training
Jorn Trommelen and Luc J. C. van Loon*
Author information ► Article notes ► Copyright and License information ► Disclaimer
This article has been cited by other articles in PMC.
Protein ingestion following resistance-type exercise stimulates muscle protein synthesis rates, and enhances the skeletal muscle adaptive response to prolonged resistance-type exercise training. As the adaptive response to a single bout of resistance exercise extends well beyond the first couple of hours of post-exercise recovery, recent studies have begun to investigate the impact of the timing and distribution of protein ingestion during more prolonged recovery periods. Recent work has shown that overnight muscle protein synthesis rates are restricted by the level of amino acid availability. Protein ingested prior to sleep is effectively digested and absorbed, and thereby stimulates muscle protein synthesis rates during overnight recovery. When applied during a prolonged period of resistance-type exercise training, protein supplementation prior to sleep can further augment gains in muscle mass and strength. Recent studies investigating the impact of pre-sleep protein ingestion suggest that at least 40 g of protein is required to display a robust increase in muscle protein synthesis rates throughout overnight sleep. Furthermore, prior exercise allows more of the pre-sleep protein-derived amino acids to be utilized for de novo muscle protein synthesis during sleep. In short, pre-sleep protein ingestion represents an effective dietary strategy to improve overnight muscle protein synthesis, thereby improving the skeletal muscle adaptive response to exercise training.
Keywords: sleep, recovery, exercise, hypertrophy, casein
A single session of exercise stimulates muscle protein synthesis rates, and to a lesser extent, muscle protein breakdown rates [1,2]. However, the muscle protein net balance will remain negative in the absence of food intake [2]. Protein ingestion stimulates muscle protein synthesis and inhibits muscle protein breakdown rates, resulting in net muscle protein accretion during the acute stages of post-exercise recovery [3]. Therefore, post-exercise protein ingestion is widely applied as a strategy to augment post-exercise muscle protein synthesis rates and, as such, to facilitate the skeletal muscle adaptive response to exercise training. Various factors have been identified which can modulate the post-exercise muscle protein synthetic response to exercise including the amount [4,5], type [6,7], timing [8], and distribution [9] of protein ingestion.
Only few studies have investigated the dose-response relationship between protein ingestion and post-exercise muscle protein synthesis rates in young [4,5] and older adults [10,11,12]. Ingestion of 20 g egg or whey protein has been shown sufficient to maximize muscle protein synthesis rates during recovery from lower-body resistance-type exercise in young males [4,5]. More recent evidence indicates that this dose-response relationship may depend on the amount of muscle tissue that was recruited during exercise, with the ingestion of 40 g protein further increasing muscle protein synthesis rates during recovery from whole-body resistance-type exercise [13].
A large variety of dietary protein sources have been shown to stimulate post-exercise muscle protein synthesis rates, including egg protein [4], whey and casein protein [14], milk and beef protein [15], and soy protein [6]. However, dietary protein sources can differ in their capacity to stimulate muscle protein synthesis rates, which appears to be largely dependent on differences in protein digestion and absorption kinetics [14,16] and amino acid composition [6,17], with the leucine content being of particular relevance [18,19].
Besides the amount and type of ingested protein, the timing and distribution of protein ingestion throughout the day can modulate post-exercise muscle protein synthesis rates. An even distribution of total protein intake over the three main meals stimulates 24 h muscle protein synthesis rates more effectively than an unbalanced distribution in which the majority (>60%) of total daily protein intake is consumed at the evening meal [20]. During 12 h of post-exercise recovery, an intermediate pattern of protein ingestion (20 g every 3 h) seems to increase muscle protein synthesis rates to a greater extent than the same amount of protein provided in less frequent but larger amounts (40 g every 6 h), or in more frequent, smaller amounts (10 g every 6 h) [9]. Therefore, an effective pattern of daily protein intake distribution to support muscle protein synthesis is to provide at least 20 g of protein with each main meal with no more than 4–5 h between meals.
As overnight sleep is typically the longest post-absorptive period during the day, we have recently introduced the concept of protein ingestion prior to sleep as a means to augment post-exercise overnight muscle protein synthesis. The aim of this review is to discuss the current state of evidence regarding the efficacy of pre-sleep protein ingestion to stimulate overnight muscle reconditioning.
In general, most studies assess the effects of food intake on the muscle protein synthetic response to exercise performed in an overnight fasted state. Such post-absorptive conditions differ from normal everyday practice in which recreational sports activities are often performed in the late afternoon or evening after a full day of habitual physical activity and food intake. Therefore, we evaluated the impact of exercise performed in a fed state in the evening and the efficacy of protein ingestion immediately after exercise on muscle protein synthesis during prolonged overnight recovery [21]. The ingestion of 20–25 g of protein during exercise increased muscle protein synthesis rates during exercise, but we observed no increase in muscle protein synthesis rates during the prolonged overnight recovery period. Muscle protein synthesis rates during overnight sleep were unexpectedly low, with values being even lower than those typically observed in the in the morning following an overnight fast. Thus, a day of habitual food intake and the ingestion of 20–25 g of protein during and/or immediately after an exercise bout performed in the evening does not suffice to augment overnight muscle protein reconditioning.
As overnight muscle protein synthesis rates are surprisingly low [21], we questioned whether they are limited by overnight plasma amino acid availability. Therefore, we hypothesized that protein provision during sleep increases overnight plasma amino acid availability and stimulates overnight muscle protein synthesis rates. As human intestinal motility follows a circadian rhythm with reduced activity during the night [22], we first assessed whether dietary protein provision during sleep leads to proper dietary protein digestion and amino acid absorption. In a proof-of-principle study, we first administrated specifically produced intrinsically l-[1-13C]-phenylalanine-labeled casein protein via a nasogastric tube while subjects were asleep and assessed the subsequent protein digestion and absorption kinetics [23]. We observed that administration of 40 g casein via a nasogastric tube during overnight sleep is followed by proper dietary protein digestion and absorption kinetics, thereby increasing overnight plasma amino acid availability and increasing muscle protein synthesis rates. Clearly, these data demonstrated that the gut functions properly at night and that protein provided during sleep strongly increases overnight muscle protein synthesis rates.
Our observation that protein administered during sleep is effectively digested and absorbed provided proof-of-principle that the gut functions properly during sleep [23]. However, nasogastric tube feeding does not represent a feasible feeding strategy for athletes. Therefore, our next step was to assess if protein ingestion prior to sleep would represent an effective dietary strategy to increase muscle protein synthesis rates during overnight post-exercise recovery [24]. Therefore, we studied recreational athletes during overnight recovery from a single bout of resistance-type exercise performed in the evening after a full day of dietary standardization. Immediately after exercise, all athletes ingested a recovery drink containing 20 g protein to maximize muscle protein synthesis rates during the acute stages of post-exercise recovery [4,24]. As explained above, this prescribed recovery strategy does not suffice to maintain elevated muscle protein synthesis rates during more prolonged overnight sleep [21]. Therefore, we provided subjects with either 40 g casein protein or a placebo drink immediately prior to sleep. In line with intragastric protein administration during sleep [23], the bolus of protein ingested prior to sleep was properly digested and absorbed throughout overnight sleep. The greater plasma amino acid availability following pre-sleep protein ingestion improved the overnight whole-body protein balance, allowing the net protein balance to become positive. In line, muscle protein synthesis rates were approximately 22% higher during overnight recovery when protein was ingested prior to sleep when compared to the placebo treatment. From these data we concluded that pre-sleep protein ingestion represents an effective dietary strategy to further augment the skeletal muscle adaptive response to resistance-type exercise training (Figure 1).
Figure 1
Schematic representation of the process of muscle protein synthesis (MPS) and muscle protein breakdown (MPB) throughout the day. Protein ingestion stimulates MPS rates and allows for net muscle protein accretion (green areas). During post-absorptive conditions, MPB rates exceed MPS rates, resulting in a net loss of muscle protein (red areas). Overnight sleep is the longest post-absorptive period of the day (A). Pre-sleep protein ingestion stimulates overnight muscle protein synthesis rates (B), thereby improving muscle reconditioning during overnight sleep.
To test this hypothesis, we assessed the impact of pre-sleep protein feeding to facilitate the skeletal muscle adaptive response to prolonged resistance-type exercise training [25]. Specifically, we selected healthy young men to participate in a 12-week resistance-type exercise training program (three exercise sessions per week) during which they ingested either 27.5 g of protein prior to sleep, or a non-caloric placebo. Muscle mass and strength increased to a greater extent in the group that ingested protein prior to sleep. These results indicate that protein supplementation prior to sleep represents an effective dietary strategy to augment the gains in muscle mass and strength during resistance-type exercise training. It remains to be established what dose and type of pre-sleep protein should be used to further optimize overnight muscle protein synthesis rates and, as such, can support greater gains in muscle mass and strength.
It should be noted that the ingestion of the pre-sleep protein supplement in both our acute and long-term studies was compared with a non-protein placebo, and not compared with protein supplementation provided at other time points. Therefore, we can only speculate on the surplus benefits of pre-sleep protein provision when compared to other time points. It can be speculated that the greater gains in muscle mass and strength are, at least partly, attributed to the pre-sleep timing of the protein supplement, as the vast majority of studies in which protein has been supplemented immediately before and/or after exercise do not show an increase in muscle mass gains when compared to a placebo [26]. However, it has been suggested that protein supplementation increases muscle mass gains mainly as a function of increased total protein intake, rather than the specific timing of a protein supplement [27,28]. As a meta-analysis was required to demonstrate that additional protein intake augments training-induced muscle hypertrophy [26], it seems unlikely that a possible positive effect of protein timing (i.e., protein supplementation at a time point compared to protein supplementation at different time point) on muscle mass gains can be detected in a longitudinal study. While it is currently unclear whether pre-sleep protein ingestion is superior to protein ingestion at a different time point, we propose that a more relevant question is whether pre-sleep protein ingestion is additive to protein intake earlier in the day. We suggest that athletes should aim to ingest sufficient protein intake at every meal to maximize muscle protein synthesis until the next meal. We have recently shown that the ingestion of large amounts of protein in the early post-exercise recovery phase does not compromise the muscle protein synthetic response to protein ingestion at a later stage [29]. This suggests that every meal moment represents a unique opportunity to stimulate muscle protein synthesis and that the muscle protein synthetic response to each meal may be additive. In addition, we have recently shown that athletes typically consume well above 1.2 g protein/kg/day, with the majority of protein consumed during the three main meals, and only a small amount of protein eaten as an evening snack (~7 g) [30]. As such, additional pre-sleep protein ingestion represents a practical strategy to increase the total daily protein intake, add another meal moment, and increase the overnight muscle protein synthesis rates; this effect is likely additive to muscle protein synthesis rates observed throughout the day.
While we have identified the overnight sleeping period as a new window of opportunity to augment post-exercise training adaptations, it remains to be established how we can maximize the impact of pre-sleep protein feeding on overnight muscle protein synthesis rates. Previously we have shown that the ingestion of 40 g protein prior to sleep stimulates overnight muscle protein rates [24], which is considerably more than the 20 g of protein that is supposed to maximize muscle protein synthesis rates during the first few hours of post-exercise recovery [4,5]. Therefore, we questioned if a more moderate amount of protein would suffice to augment overnight muscle protein synthesis rates. To address this issue, we performed a follow-up study similar in design to our previous pre-sleep protein work, with the main difference that we provided 30 g of highly enriched intrinsically labeled protein prior to sleep, with or without an additional 2 g of free leucine. In contrast to our previous findings with 40 g protein, the ingestion of 30 g protein prior to sleep did not significantly increase overnight muscle protein synthesis rates (preliminary observations). This suggests that a pre-sleep protein dose-response relationship exists, which differs from the immediate post-exercise recovery period during which the ingestion of merely 20 g protein seems to maximize post-exercise muscle protein synthesis rates in young adults.
The ingestion of highly enriched, intrinsically l-[1-13C]-phenylalanine-labeled protein allowed us to also directly assess the metabolic fate of the pre-sleep dietary protein-derived amino acids. Pre-sleep protein-derived l-[1-13C]-phenylalanine was incorporated in de novo muscle protein as evidenced by the increase in muscle protein–bound l-[1-13C]-phenylalanine following overnight recovery, demonstrating that the pre-sleep protein provided amino acids as precursors for de novo myofibrillar protein accretion during overnight sleep. This provides mechanistic evidence to support our observation that the ingestion of 30 g protein prior to sleep augments muscle mass during three months of resistance-type exercise training [25]. However, our data suggest that at least 40 g of pre-sleep protein is required to induce a more substantial, detectable increase in muscle protein synthesis rates when assessed acutely over a 7.5 h overnight period.
As we anticipated that 30 g of pre-sleep protein might not be sufficient to adequately increase overnight muscle protein synthesis rates, we included a third treatment in which 2 g crystalline leucine was added to the 30 g bolus of protein. The addition of supplemental free leucine to a suboptimal amount of protein has been shown to enhance post-exercise muscle protein synthesis rates [18,19,31,32]. Despite these previous observations, co-ingesting free leucine with 30 g of casein prior to sleep did not augment the overnight muscle protein synthetic response. Given the extended duration of overnight sleep compared to a typical postprandial period (8 vs. 4–5 h), it is tempting to speculate that larger amounts of protein (≥40 g) are required to maximize muscle protein synthesis rates during overnight sleep.
It has been well established that the muscle protein synthetic response to protein ingestion is enhanced following exercise when exercise is performed in the morning following an overnight fast [12,33]. Recently, we evaluated the effect of resistance-type exercise performed in the evening on the muscle protein synthetic response to pre-sleep protein ingestion [34]. Postprandial overnight muscle protein synthesis rates were higher when exercise had been performed earlier that evening and more of the ingested protein-derived amino acids were directed towards de novo myofibrillar protein synthesis during overnight sleep. Therefore, protein ingestion prior to sleep represents an effective strategy to enhance overnight muscle reconditioning and is likely of even more relevance on exercise training days. In line, we have shown that physical activity performed in the evening increases the overnight muscle protein synthetic response to pre-sleep protein ingestion in older adults [35]. Clearly, combing pre-sleep protein ingestion with resistance-type exercise represents a more effective strategy to further enhance overnight skeletal muscle protein synthesis rates and increases the efficiency by which dietary protein is used for muscle protein accretion (Figure 2).
Figure 2
Conceptual framework of the overnight muscle protein synthetic response to 40 g of pre-sleep protein feeding at rest or following prior exercise.
As protein sources differ in their capacity to stimulate muscle protein synthesis, the type of protein ingested prior to sleep may modulate the overnight muscle protein synthetic response. So far, all studies assessing the efficacy of pre-sleep protein ingestion on exercise reconditioning have provided casein protein. Casein is a more slowly digestible protein source, allowing a more moderate but prolonged rise in plasma amino acid concentrations [17]. Given the extended nature of overnight sleep, it could be speculated that such a more sustained postprandial aminoacidemia during overnight sleep is preferred as it will provide precursors to support muscle protein synthesis rates throughout the entire night. In contrast, whey protein is a more rapidly digestible protein, resulting in a pronounced but transient rise in plasma amino acid concentrations [17]. Ingestion of a single bolus of whey protein has been shown to stimulate muscle protein synthesis rates to a greater degree than casein protein when assessed over periods up to 6 h [6,17,36]. This has been attributed to the more rapid protein digestion and amino acid absorption kinetics as well as the higher leucine content in whey versus casein protein, resulting in a more rapid rise in postprandial plasma leucine concentrations [37]. It remains to be established if whey is superior to casein protein when ingested prior to sleep and muscle protein synthesis rates are assessed over a more prolonged overnight period of 7.5 h. The plasma levels of leucine do not seem to be the only factor in this regard, as we recently did not observe any differences in overnight muscle protein synthesis rates following the ingestion of 30 g casein with or without 2 g crystalline leucine (preliminary observations). Snijders et al. [25] provided a casein protein supplement that consisted of 50% micellar casein and 50% casein hydrolysate. When casein protein is hydrolyzed, its digestion and absorption properties resemble a more rapid digestible protein [38]. Therefore, pre-sleep ingestion of a mixture of a slow and more rapidly digestible protein source appears to be effective to augment muscle mass and strength gains during a prolonged resistance-type exercise program. We speculate that a variety of high-quality animal-based protein sources can augment overnight muscle protein synthesis rates when provided in sufficient amounts (≥40 g; Table 1), with relatively minor differences in efficacy between sources.
Table 1
Quantity of protein sources to provide 40 g pre-sleep protein.
Overnight sleep has emerged as a novel window of opportunity to modulate muscle protein metabolism. Pre-sleep protein ingestion represents an effective dietary strategy to stimulate both the acute and long-term skeletal muscle adaptive response to resistance-type exercise training [24,25]. There are numerous other potential applications of protein ingestion prior to sleep. Protein ingestion prior to sleep may also enhance exercise training adaptations to other exercise modalities. However, research on the impact of protein supplementation on other modes of exercise such as concurrent training [39] or endurance-type exercise training [40] is surprisingly scarce. While protein ingested immediately after endurance-type exercise does not appear to further augment mitochondrial protein synthesis rates [40], amino acid administration at rest stimulates mitochondrial protein synthesis rates [41]. It remains to be established if pre-sleep protein can augment the adaptive response to endurance-type exercise training with greater increases in skeletal muscle oxidative capacity, vascular density and/or endurance performance capacity.
Protein administration during sleep has been shown to stimulate overnight muscle protein synthesis rates in older adults [23]. Consequently, pre-sleep protein feeding may also represent an effective interventional strategy to support muscle mass maintenance in the older population or possibly even in patients in more clinically compromised conditions characterized by accelerated muscle loss such as acute sickness, systematic inflammation, and muscle disuse [42,43].
Muscle protein synthesis rates are particularly low during sleep, even when 20 g protein is ingested immediately after exercise performed in the evening. Protein ingested immediately prior to sleep is effectively digested and absorbed, thereby increasing amino acid availability during overnight sleep. Greater amino acid availability during sleep stimulates muscle protein synthesis rates and improves whole-body protein net balance during overnight recovery. At least 40 g of dietary protein should be ingested prior to sleep to elicit a robust stimulation of muscle protein synthesis rates throughout the night. Resistance-type exercise performed during the day augments the overnight muscle protein synthetic response to pre-sleep protein ingestion and allows more of the protein-derived amino acids to be used as precursors for de novo muscle protein synthesis. When applied during prolonged resistance-type exercise, pre-sleep protein supplementation can be used effectively to further increase gains in muscle mass and strength.
I just wanted to put this out there, because to many bro's advocate-
"Long as you hit your required protein macros before bed then there is no need to take it before bed.""
- Not entirely true, but there's some truth to that, but other benefits also exist with slow absorption proteins while resting/recovering/sleep
There is some absolute truth with consuming slow/fast acting proteins.
The speed of absorption of dietary amino acids varies according to the type of ingested dietary protein... Learn just how important slow and fast proteins, taken at the appropriate time, can affect your ability to put on lean mass.Many people avoid eating right before bed as they fear that the calories are more likely to be stored as fat. This is not the case though. Your body doesn't have an on-off switch and you still burn calories while you sleep. According to the American Dietetic Association, it's excess calories that determine whether you gain weight, not when you eat them. Too many calories at breakfast or lunch will be just as detrimental as too many calories right before bed.
Premise
As much as we may think of bodybuilding as a cloistered subculture, we are forever bombarded with training and nutritional tips from sources far removed from squat racks and posing daises. So it is with this axiom, which is such a ubiquitous feature of the sort of diets Oprah hypes that many beginning bodybuilders dare not breach it, and it breeds confusion about what and when to eat to gain only muscle and not fat
Science
When you sleep, you?re on a fast. During that fast, your body is forced to turn to your own muscle protein for fuel, converting those amino acids into glucose. In other words, while you?re in dreamland, you?re experiencing the nightmare of cannibalizing your own muscles. The longer you go before sleep without eating, the more your muscle will be eaten away. That?s why we always recommend that you end your day with a slow-digesting protein, such as a casein protein shake or cottage cheese.
(Research from Groups located in Texas, and even the Netherlands discovered that trained bodybuilders drinking a casein protein shake right before bed for eight weeks gained significantly more muscle than those who consumed the same casein shake in the middle of the day.)
Verdict
We started with the easiest myth to shoot down, for not only is it OK to chow down long after sundown, it?s crucial to eat a protein meal immediately before going to bed in order to feed your muscles the nutrients they need to recover and grow while you sleep. Go with 20-40 grams of slow-digesting protein, such as a casein shake or cottage cheese. If you?re trying to pack on mass and don?t store fat easily, take your protein with about 20-40 g of slow-digesting carbs, such as oatmeal, sweet potatoes or whole-wheat bread.
Now with this being said:
Read the study and see the charts in regards to why a slow releasing protein has a full advantage (pre-sleep)
Slow and fast dietary proteins differently modulate postprandial protein accretion. Boirie Y, et al. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14930-5.Full text at:
Proc. Natl. Acad. Sci. USA
Vol. 94, pp. 14930?14935, December 1997
Physiology
Slow and fast dietary proteins differently modulate postprandial
protein accretion
(amino acid turnoverypostprandial protein anabolismymilk proteinystable isotopes)
YVES BOIRIE*, MARTIAL DANGIN*?, PIERRE GACHON*, MARIE-PAULE VASSON?, JEAN-LOUIS MAUBOIS?,
AND BERNARD BEAUFRE`RE*?
*Laboratoire de Nutrition Humaine, Universite? Clermont Auvergne, Centre de Recherche en Nutrition Humaine, BP 321, 63009 Clermont-Ferrand Cedex 1,
France; ?Nestec, Ltd., Nestle? Research Center, P.O. Box 44, CH 1000 Lausanne 26, Switzerland; ?Laboratoire de Biochimie, Biologie Mole?culaire et Nutrition,
Universite? Clermont Auvergne, BP 38, 63001 Clermont-Ferrand Cedex 1, France; and ?Laboratoire de Technologie Laitie`re, Institut National de la Recherche
Agronomique, 35042 Rennes Cedex, France
Communicated by John Waterlow, University of London, London, United Kingdom, October 7, 1997 (received for review April 20, 1997)
In relation to the 1997 Boirie study, Lyle summarized that the researchers found the following: whey spiked blood amino acid levels faster than casein, but blood amino acid levels dropped more quickly as well. Casein, in contrast, took much longer to digest, actually providing amino acids for around 8 hours to the body ... Both casein and whey hit the bloodstream at about the same time (about an hour in), that is, whey didn?t actually get into the system faster. However, whey spiked blood amino acid levels higher at that one hour point.


ABSTRACT The speed of absorption of dietary amino
acids by the gut varies according to the type of ingested dietary
protein. This could affect postprandial protein synthesis,
breakdown, and deposition. To test this hypothesis, two intrinsically
13C-leucine-labeled milk proteins, casein (CAS)
and whey protein (WP), of different physicochemical properties
were ingested as one single meal by healthy adults.
Postprandial whole body leucine kinetics were assessed by
using a dual tracer methodology. WP induced a dramatic but
short increase of plasma amino acids. CAS induced a prolonged
plateau of moderate hyperaminoacidemia, probably
because of a slow gastric emptying. Whole body protein
breakdown was inhibited by 34% after CAS ingestion but not
after WP ingestion. Postprandial protein synthesis was stimulated
by 68% with the WP meal and to a lesser extent (131%)
with the CAS meal. Postprandial whole body leucine oxidation
over 7 h was lower with CAS (272 6 91 mmolzkg21) than with
WP (373 6 56 mmolzkg21). Leucine intake was identical in
both meals (380 mmolzkg21). Therefore, net leucine balance
over the 7 h after the meal was more positive with CAS than
with WP (P < 0.05, WP vs. CAS). In conclusion, the speed of
protein digestion and amino acid absorption from the gut has
a major effect on whole body protein anabolism after one
single meal. By analogy with carbohydrate metabolism, slow
and fast proteins modulate the postprandial metabolic response,
a concept to be applied to wasting situations.
Dietary carbohydrates are commonly classified as slow and fast
because it now is well recognized that their structure affects
their speed of absorption, which in turn has a major impact on
the metabolic and hormonal response to a meal (1). On the
other hand, little is known about whether postprandial protein
kinetics are affected by the speed of absorption of dietary
amino acids; the latter is very variable, depending on gastric
and intestinal motility, luminal digestion, and finally mucosal
absorption. This lack of data is due to the fact that postprandial
amino acid kinetics have been studied almost exclusively
during continuous feeding, obtained either by a nasogastric
infusion or by small repeated meals (2?7). Measurements are
done 2?4 h after initiation of feeding, once isotopic and
substrate steady-state is achieved. Under these conditions, any
difference related to the speed of dietary amino acid absorption
is blunted.
There is, however, indirect evidence that this parameter
might be of importance. Indeed, the postprandial amino acid
levels differ a lot depending on the mode of administration of
a dietary protein; a single protein meal results in an acute but
transient peak of amino acids (9?11) whereas the same amount
of the same protein given in a continuous manner, which
mimics a slow absorption, induces a smaller but prolonged
increase (12). Amino acids are potent modulators of protein
synthesis, breakdown, and oxidation, so such different patterns
of postprandial amino acidemia might well result in different
postprandial protein kinetics and gain. Of interest, whole body
leucine balance, an index of protein deposition, was shown
recently to differ under these two circumstances (13).
Therefore, our hypothesis was that the speed of absorption
by the gut of amino acids derived from dietary proteins might
affect whole body protein synthesis, breakdown, and oxidation,
which in turn control protein deposition. To test this hypothesis,
we compared those parameters, assessed by leucine
kinetics, after ingestion of a single meal containing either whey
protein (WP) or casein (CAS), taken as paradigms for ??fast??
and ??slow?? proteins, respectively. Indeed, WP is a soluble
protein whereas CAS clots into the stomach, which delays its
gastric emptying and thus probably results in a slower release
of amino acids (14). Speed of amino acid absorption was
directly assessed by using a newly developed tracer, i.e., milk
protein fractions intrinsically labeled with L-[1-13C]leucine
(15). Leucine kinetics were modelized by using non-steadystate
equations as recently described (16). Our results demonstrate
that amino acids derived from CAS are indeed slowly
released from the gut and that slow and fast proteins differently
modulate postprandial changes of whole body protein
synthesis, breakdown, oxidation, and deposition.
I just wanted to post some of the study.. I will include the hyper link below that provides full detail,in regards to fast/slow proteins/and aminos..
(Slow and fast dietary proteins differently modulate postprandial protein)
Now with this being said more reads in regards-
ne)levels peak during sleep. 75 percent of daily hGH output is produced nse to get all the nutrients you'll need, then let the GH do
tNow what protein is the best to
consume prior to sleeping?
"Casein "
Keep reading
What is casein protein?
Casein (pronounced kay-seen) is the predominant protein found in milk. It is made by separating the casein from the whey in dairy (milk protein is 80 percent casein and 20 percent whey). There are three main types of casein protein: micellar casein, milk protein isolate and calcium caseinate. On average, one scoop (30 grams) of casein protein powder has approximately 100-120 calories and 25 grams of protein.
Besides its slow-digesting benefits, casein is invaluable for its high glutamine content. Of all the protein powders available, casein has the highest concentration of this amino acid. Glutamine provides a multitude of functions, which include increasing levels of the branched chain amino acid leucine in muscle fibers, enhancing protein synthesis and therefore, muscle growth. Because the immune system requires glutamine to function, consuming extra glutamine prevents the immune system from stealing it from muscle fibers, further averting catabolism. Glutamine also boosts growth hormone levels and can even aid fat loss by increasing the amount of calories and body fat burned both at rest and during exercise.
Do blended proteins such as whey offer the same effect as straight casein?
Either protein supplements are straight whey, soy, egg or casein; or they are a combination of any or all of these kinds of proteins, making them blends. What can a blend of proteins offer that a straight protein cannot? Basically, different rates of digestion. This means you can take a blended protein any time to get quick, medium, and prolonged absorption of protein.
But, I really like my ?whey? protein supplement.
Turn your favorite whey protein shake into a slow digesting one by simply mixing with milk, preferably low fat or skimmed. While casein protein is ?optimal? before bed, don?t forget that milk is 80 percent casein, adding it to any whey protein will slow down its absorption. Adding a fat such as natural peanut butter, flax or other healthy fat can further slow digestion, thus ?mimicking? casein protein.
To close the read and get to the conclusion here..
Don't deprive yourself, and fall for the hoopla that if you consume your proper intake of macro's that you don't need protein prior to rest/recovery(sleep)..
It most certainly won't hinder your gains on a large scale,but it surely is more beneficial than going without!
Regards,
Vision
____________________________
More info
BCAA's before bed is it necessary?
Truth is BCAA's need to be avail for use along with protein many researches
have stated that low to no protein before bed yields a low protein synthesis rate while sleeping,
you do burn cals when sleeping, you ever wake up lighter in the AM???
Its been proven that it will yield an add advantage with keeping plasma BCAA's levels higher throughout the state of rest/sleep/night
Pre-Sleep Protein Ingestion to Improve the Skeletal Muscle Adaptive Response to Exercise Training
Jorn Trommelen and Luc J. C. van Loon*
Author information ► Article notes ► Copyright and License information ► Disclaimer
This article has been cited by other articles in PMC.
AbstractProtein ingestion following resistance-type exercise stimulates muscle protein synthesis rates, and enhances the skeletal muscle adaptive response to prolonged resistance-type exercise training. As the adaptive response to a single bout of resistance exercise extends well beyond the first couple of hours of post-exercise recovery, recent studies have begun to investigate the impact of the timing and distribution of protein ingestion during more prolonged recovery periods. Recent work has shown that overnight muscle protein synthesis rates are restricted by the level of amino acid availability. Protein ingested prior to sleep is effectively digested and absorbed, and thereby stimulates muscle protein synthesis rates during overnight recovery. When applied during a prolonged period of resistance-type exercise training, protein supplementation prior to sleep can further augment gains in muscle mass and strength. Recent studies investigating the impact of pre-sleep protein ingestion suggest that at least 40 g of protein is required to display a robust increase in muscle protein synthesis rates throughout overnight sleep. Furthermore, prior exercise allows more of the pre-sleep protein-derived amino acids to be utilized for de novo muscle protein synthesis during sleep. In short, pre-sleep protein ingestion represents an effective dietary strategy to improve overnight muscle protein synthesis, thereby improving the skeletal muscle adaptive response to exercise training.
Keywords: sleep, recovery, exercise, hypertrophy, casein
1. IntroductionA single session of exercise stimulates muscle protein synthesis rates, and to a lesser extent, muscle protein breakdown rates [1,2]. However, the muscle protein net balance will remain negative in the absence of food intake [2]. Protein ingestion stimulates muscle protein synthesis and inhibits muscle protein breakdown rates, resulting in net muscle protein accretion during the acute stages of post-exercise recovery [3]. Therefore, post-exercise protein ingestion is widely applied as a strategy to augment post-exercise muscle protein synthesis rates and, as such, to facilitate the skeletal muscle adaptive response to exercise training. Various factors have been identified which can modulate the post-exercise muscle protein synthetic response to exercise including the amount [4,5], type [6,7], timing [8], and distribution [9] of protein ingestion.
Only few studies have investigated the dose-response relationship between protein ingestion and post-exercise muscle protein synthesis rates in young [4,5] and older adults [10,11,12]. Ingestion of 20 g egg or whey protein has been shown sufficient to maximize muscle protein synthesis rates during recovery from lower-body resistance-type exercise in young males [4,5]. More recent evidence indicates that this dose-response relationship may depend on the amount of muscle tissue that was recruited during exercise, with the ingestion of 40 g protein further increasing muscle protein synthesis rates during recovery from whole-body resistance-type exercise [13].
A large variety of dietary protein sources have been shown to stimulate post-exercise muscle protein synthesis rates, including egg protein [4], whey and casein protein [14], milk and beef protein [15], and soy protein [6]. However, dietary protein sources can differ in their capacity to stimulate muscle protein synthesis rates, which appears to be largely dependent on differences in protein digestion and absorption kinetics [14,16] and amino acid composition [6,17], with the leucine content being of particular relevance [18,19].
Besides the amount and type of ingested protein, the timing and distribution of protein ingestion throughout the day can modulate post-exercise muscle protein synthesis rates. An even distribution of total protein intake over the three main meals stimulates 24 h muscle protein synthesis rates more effectively than an unbalanced distribution in which the majority (>60%) of total daily protein intake is consumed at the evening meal [20]. During 12 h of post-exercise recovery, an intermediate pattern of protein ingestion (20 g every 3 h) seems to increase muscle protein synthesis rates to a greater extent than the same amount of protein provided in less frequent but larger amounts (40 g every 6 h), or in more frequent, smaller amounts (10 g every 6 h) [9]. Therefore, an effective pattern of daily protein intake distribution to support muscle protein synthesis is to provide at least 20 g of protein with each main meal with no more than 4–5 h between meals.
As overnight sleep is typically the longest post-absorptive period during the day, we have recently introduced the concept of protein ingestion prior to sleep as a means to augment post-exercise overnight muscle protein synthesis. The aim of this review is to discuss the current state of evidence regarding the efficacy of pre-sleep protein ingestion to stimulate overnight muscle reconditioning.
2. Overnight Protein MetabolismIn general, most studies assess the effects of food intake on the muscle protein synthetic response to exercise performed in an overnight fasted state. Such post-absorptive conditions differ from normal everyday practice in which recreational sports activities are often performed in the late afternoon or evening after a full day of habitual physical activity and food intake. Therefore, we evaluated the impact of exercise performed in a fed state in the evening and the efficacy of protein ingestion immediately after exercise on muscle protein synthesis during prolonged overnight recovery [21]. The ingestion of 20–25 g of protein during exercise increased muscle protein synthesis rates during exercise, but we observed no increase in muscle protein synthesis rates during the prolonged overnight recovery period. Muscle protein synthesis rates during overnight sleep were unexpectedly low, with values being even lower than those typically observed in the in the morning following an overnight fast. Thus, a day of habitual food intake and the ingestion of 20–25 g of protein during and/or immediately after an exercise bout performed in the evening does not suffice to augment overnight muscle protein reconditioning.
3. Does the Gut Function at Night?As overnight muscle protein synthesis rates are surprisingly low [21], we questioned whether they are limited by overnight plasma amino acid availability. Therefore, we hypothesized that protein provision during sleep increases overnight plasma amino acid availability and stimulates overnight muscle protein synthesis rates. As human intestinal motility follows a circadian rhythm with reduced activity during the night [22], we first assessed whether dietary protein provision during sleep leads to proper dietary protein digestion and amino acid absorption. In a proof-of-principle study, we first administrated specifically produced intrinsically l-[1-13C]-phenylalanine-labeled casein protein via a nasogastric tube while subjects were asleep and assessed the subsequent protein digestion and absorption kinetics [23]. We observed that administration of 40 g casein via a nasogastric tube during overnight sleep is followed by proper dietary protein digestion and absorption kinetics, thereby increasing overnight plasma amino acid availability and increasing muscle protein synthesis rates. Clearly, these data demonstrated that the gut functions properly at night and that protein provided during sleep strongly increases overnight muscle protein synthesis rates.
4. Pre-Sleep Protein Feeding as a Strategy to Increase Overnight Muscle Protein SynthesisOur observation that protein administered during sleep is effectively digested and absorbed provided proof-of-principle that the gut functions properly during sleep [23]. However, nasogastric tube feeding does not represent a feasible feeding strategy for athletes. Therefore, our next step was to assess if protein ingestion prior to sleep would represent an effective dietary strategy to increase muscle protein synthesis rates during overnight post-exercise recovery [24]. Therefore, we studied recreational athletes during overnight recovery from a single bout of resistance-type exercise performed in the evening after a full day of dietary standardization. Immediately after exercise, all athletes ingested a recovery drink containing 20 g protein to maximize muscle protein synthesis rates during the acute stages of post-exercise recovery [4,24]. As explained above, this prescribed recovery strategy does not suffice to maintain elevated muscle protein synthesis rates during more prolonged overnight sleep [21]. Therefore, we provided subjects with either 40 g casein protein or a placebo drink immediately prior to sleep. In line with intragastric protein administration during sleep [23], the bolus of protein ingested prior to sleep was properly digested and absorbed throughout overnight sleep. The greater plasma amino acid availability following pre-sleep protein ingestion improved the overnight whole-body protein balance, allowing the net protein balance to become positive. In line, muscle protein synthesis rates were approximately 22% higher during overnight recovery when protein was ingested prior to sleep when compared to the placebo treatment. From these data we concluded that pre-sleep protein ingestion represents an effective dietary strategy to further augment the skeletal muscle adaptive response to resistance-type exercise training (Figure 1).
Figure 1
Schematic representation of the process of muscle protein synthesis (MPS) and muscle protein breakdown (MPB) throughout the day. Protein ingestion stimulates MPS rates and allows for net muscle protein accretion (green areas). During post-absorptive conditions, MPB rates exceed MPS rates, resulting in a net loss of muscle protein (red areas). Overnight sleep is the longest post-absorptive period of the day (A). Pre-sleep protein ingestion stimulates overnight muscle protein synthesis rates (B), thereby improving muscle reconditioning during overnight sleep.
To test this hypothesis, we assessed the impact of pre-sleep protein feeding to facilitate the skeletal muscle adaptive response to prolonged resistance-type exercise training [25]. Specifically, we selected healthy young men to participate in a 12-week resistance-type exercise training program (three exercise sessions per week) during which they ingested either 27.5 g of protein prior to sleep, or a non-caloric placebo. Muscle mass and strength increased to a greater extent in the group that ingested protein prior to sleep. These results indicate that protein supplementation prior to sleep represents an effective dietary strategy to augment the gains in muscle mass and strength during resistance-type exercise training. It remains to be established what dose and type of pre-sleep protein should be used to further optimize overnight muscle protein synthesis rates and, as such, can support greater gains in muscle mass and strength.
It should be noted that the ingestion of the pre-sleep protein supplement in both our acute and long-term studies was compared with a non-protein placebo, and not compared with protein supplementation provided at other time points. Therefore, we can only speculate on the surplus benefits of pre-sleep protein provision when compared to other time points. It can be speculated that the greater gains in muscle mass and strength are, at least partly, attributed to the pre-sleep timing of the protein supplement, as the vast majority of studies in which protein has been supplemented immediately before and/or after exercise do not show an increase in muscle mass gains when compared to a placebo [26]. However, it has been suggested that protein supplementation increases muscle mass gains mainly as a function of increased total protein intake, rather than the specific timing of a protein supplement [27,28]. As a meta-analysis was required to demonstrate that additional protein intake augments training-induced muscle hypertrophy [26], it seems unlikely that a possible positive effect of protein timing (i.e., protein supplementation at a time point compared to protein supplementation at different time point) on muscle mass gains can be detected in a longitudinal study. While it is currently unclear whether pre-sleep protein ingestion is superior to protein ingestion at a different time point, we propose that a more relevant question is whether pre-sleep protein ingestion is additive to protein intake earlier in the day. We suggest that athletes should aim to ingest sufficient protein intake at every meal to maximize muscle protein synthesis until the next meal. We have recently shown that the ingestion of large amounts of protein in the early post-exercise recovery phase does not compromise the muscle protein synthetic response to protein ingestion at a later stage [29]. This suggests that every meal moment represents a unique opportunity to stimulate muscle protein synthesis and that the muscle protein synthetic response to each meal may be additive. In addition, we have recently shown that athletes typically consume well above 1.2 g protein/kg/day, with the majority of protein consumed during the three main meals, and only a small amount of protein eaten as an evening snack (~7 g) [30]. As such, additional pre-sleep protein ingestion represents a practical strategy to increase the total daily protein intake, add another meal moment, and increase the overnight muscle protein synthesis rates; this effect is likely additive to muscle protein synthesis rates observed throughout the day.
5. Pre-Sleep Protein Feeding CharacteristicsWhile we have identified the overnight sleeping period as a new window of opportunity to augment post-exercise training adaptations, it remains to be established how we can maximize the impact of pre-sleep protein feeding on overnight muscle protein synthesis rates. Previously we have shown that the ingestion of 40 g protein prior to sleep stimulates overnight muscle protein rates [24], which is considerably more than the 20 g of protein that is supposed to maximize muscle protein synthesis rates during the first few hours of post-exercise recovery [4,5]. Therefore, we questioned if a more moderate amount of protein would suffice to augment overnight muscle protein synthesis rates. To address this issue, we performed a follow-up study similar in design to our previous pre-sleep protein work, with the main difference that we provided 30 g of highly enriched intrinsically labeled protein prior to sleep, with or without an additional 2 g of free leucine. In contrast to our previous findings with 40 g protein, the ingestion of 30 g protein prior to sleep did not significantly increase overnight muscle protein synthesis rates (preliminary observations). This suggests that a pre-sleep protein dose-response relationship exists, which differs from the immediate post-exercise recovery period during which the ingestion of merely 20 g protein seems to maximize post-exercise muscle protein synthesis rates in young adults.
The ingestion of highly enriched, intrinsically l-[1-13C]-phenylalanine-labeled protein allowed us to also directly assess the metabolic fate of the pre-sleep dietary protein-derived amino acids. Pre-sleep protein-derived l-[1-13C]-phenylalanine was incorporated in de novo muscle protein as evidenced by the increase in muscle protein–bound l-[1-13C]-phenylalanine following overnight recovery, demonstrating that the pre-sleep protein provided amino acids as precursors for de novo myofibrillar protein accretion during overnight sleep. This provides mechanistic evidence to support our observation that the ingestion of 30 g protein prior to sleep augments muscle mass during three months of resistance-type exercise training [25]. However, our data suggest that at least 40 g of pre-sleep protein is required to induce a more substantial, detectable increase in muscle protein synthesis rates when assessed acutely over a 7.5 h overnight period.
As we anticipated that 30 g of pre-sleep protein might not be sufficient to adequately increase overnight muscle protein synthesis rates, we included a third treatment in which 2 g crystalline leucine was added to the 30 g bolus of protein. The addition of supplemental free leucine to a suboptimal amount of protein has been shown to enhance post-exercise muscle protein synthesis rates [18,19,31,32]. Despite these previous observations, co-ingesting free leucine with 30 g of casein prior to sleep did not augment the overnight muscle protein synthetic response. Given the extended duration of overnight sleep compared to a typical postprandial period (8 vs. 4–5 h), it is tempting to speculate that larger amounts of protein (≥40 g) are required to maximize muscle protein synthesis rates during overnight sleep.
6. Prior ExerciseIt has been well established that the muscle protein synthetic response to protein ingestion is enhanced following exercise when exercise is performed in the morning following an overnight fast [12,33]. Recently, we evaluated the effect of resistance-type exercise performed in the evening on the muscle protein synthetic response to pre-sleep protein ingestion [34]. Postprandial overnight muscle protein synthesis rates were higher when exercise had been performed earlier that evening and more of the ingested protein-derived amino acids were directed towards de novo myofibrillar protein synthesis during overnight sleep. Therefore, protein ingestion prior to sleep represents an effective strategy to enhance overnight muscle reconditioning and is likely of even more relevance on exercise training days. In line, we have shown that physical activity performed in the evening increases the overnight muscle protein synthetic response to pre-sleep protein ingestion in older adults [35]. Clearly, combing pre-sleep protein ingestion with resistance-type exercise represents a more effective strategy to further enhance overnight skeletal muscle protein synthesis rates and increases the efficiency by which dietary protein is used for muscle protein accretion (Figure 2).
Figure 2
Conceptual framework of the overnight muscle protein synthetic response to 40 g of pre-sleep protein feeding at rest or following prior exercise.
7. Type of Pre-Sleep ProteinAs protein sources differ in their capacity to stimulate muscle protein synthesis, the type of protein ingested prior to sleep may modulate the overnight muscle protein synthetic response. So far, all studies assessing the efficacy of pre-sleep protein ingestion on exercise reconditioning have provided casein protein. Casein is a more slowly digestible protein source, allowing a more moderate but prolonged rise in plasma amino acid concentrations [17]. Given the extended nature of overnight sleep, it could be speculated that such a more sustained postprandial aminoacidemia during overnight sleep is preferred as it will provide precursors to support muscle protein synthesis rates throughout the entire night. In contrast, whey protein is a more rapidly digestible protein, resulting in a pronounced but transient rise in plasma amino acid concentrations [17]. Ingestion of a single bolus of whey protein has been shown to stimulate muscle protein synthesis rates to a greater degree than casein protein when assessed over periods up to 6 h [6,17,36]. This has been attributed to the more rapid protein digestion and amino acid absorption kinetics as well as the higher leucine content in whey versus casein protein, resulting in a more rapid rise in postprandial plasma leucine concentrations [37]. It remains to be established if whey is superior to casein protein when ingested prior to sleep and muscle protein synthesis rates are assessed over a more prolonged overnight period of 7.5 h. The plasma levels of leucine do not seem to be the only factor in this regard, as we recently did not observe any differences in overnight muscle protein synthesis rates following the ingestion of 30 g casein with or without 2 g crystalline leucine (preliminary observations). Snijders et al. [25] provided a casein protein supplement that consisted of 50% micellar casein and 50% casein hydrolysate. When casein protein is hydrolyzed, its digestion and absorption properties resemble a more rapid digestible protein [38]. Therefore, pre-sleep ingestion of a mixture of a slow and more rapidly digestible protein source appears to be effective to augment muscle mass and strength gains during a prolonged resistance-type exercise program. We speculate that a variety of high-quality animal-based protein sources can augment overnight muscle protein synthesis rates when provided in sufficient amounts (≥40 g; Table 1), with relatively minor differences in efficacy between sources.
Table 1
Quantity of protein sources to provide 40 g pre-sleep protein.
| Food Item | Quantity |
|---|---|
| Cooked eggs | 7 eggs |
| Low fat milk | 5 cups (1025 mL) |
| Low fat yogurt | 5 cups (1176 mL) |
| Chicken breast | 2 breasts (176 g) |
| Steak | 2 steaks (168 g) |
| Protein concentrate in water | 3 scoops (60 g) |
| Protein concentrate in low-fat milk | 2 scoops in 300 mL |
8. ApplicationsOvernight sleep has emerged as a novel window of opportunity to modulate muscle protein metabolism. Pre-sleep protein ingestion represents an effective dietary strategy to stimulate both the acute and long-term skeletal muscle adaptive response to resistance-type exercise training [24,25]. There are numerous other potential applications of protein ingestion prior to sleep. Protein ingestion prior to sleep may also enhance exercise training adaptations to other exercise modalities. However, research on the impact of protein supplementation on other modes of exercise such as concurrent training [39] or endurance-type exercise training [40] is surprisingly scarce. While protein ingested immediately after endurance-type exercise does not appear to further augment mitochondrial protein synthesis rates [40], amino acid administration at rest stimulates mitochondrial protein synthesis rates [41]. It remains to be established if pre-sleep protein can augment the adaptive response to endurance-type exercise training with greater increases in skeletal muscle oxidative capacity, vascular density and/or endurance performance capacity.
Protein administration during sleep has been shown to stimulate overnight muscle protein synthesis rates in older adults [23]. Consequently, pre-sleep protein feeding may also represent an effective interventional strategy to support muscle mass maintenance in the older population or possibly even in patients in more clinically compromised conditions characterized by accelerated muscle loss such as acute sickness, systematic inflammation, and muscle disuse [42,43].
9. ConclusionsMuscle protein synthesis rates are particularly low during sleep, even when 20 g protein is ingested immediately after exercise performed in the evening. Protein ingested immediately prior to sleep is effectively digested and absorbed, thereby increasing amino acid availability during overnight sleep. Greater amino acid availability during sleep stimulates muscle protein synthesis rates and improves whole-body protein net balance during overnight recovery. At least 40 g of dietary protein should be ingested prior to sleep to elicit a robust stimulation of muscle protein synthesis rates throughout the night. Resistance-type exercise performed during the day augments the overnight muscle protein synthetic response to pre-sleep protein ingestion and allows more of the protein-derived amino acids to be used as precursors for de novo muscle protein synthesis. When applied during prolonged resistance-type exercise, pre-sleep protein supplementation can be used effectively to further increase gains in muscle mass and strength.
Last edited:






